The Long Haul: Engineering High-Tech Solutions to the Organ Shortage
I envision that a few years from now when I have a patient waiting for an organ, I could pick the organ from the shelf and save this patient’s life.
Carlos Esquivel, M.D., Ph.D.; Professor of Surgery; Director Pediatric Liver Transplant Program; Chief, Division of Abdominal Transplantation, Stanford University
Nearly 30 years ago, Dr. Carlos Esquivel performed a liver transplant on our one-year-old daughter Clare. Though otherwise healthy, Clare suffered from biliary atresia, a rare pediatric disease in which the liver bile ducts do not drain properly. After months on the waitlist, we received a midnight call that a liver was being flown to San Francisco for a transplant the next morning. Within two weeks of surgery the transplant failed. An emergency request went out for another liver; 36 hours later we became deeply indebted to another family 3,000 miles away. The horrific loss of their 10-year-old in a freak accident enabled Clare to have a successful second transplant. We resumed a “normal” family life. But unbeknownst to the doctors at the time, some young children are genetically predisposed to contracting a wild lymphoma-like cancer from immunosuppression drugs. Despite her well-functioning new liver, Clare died a few months later.
Clare, after two liver transplants
Aside from significant improvements in immunosuppression, much of the transplant experience — waiting for a suitable organ while the patient deteriorates to the point of organ failure — has not changed. Scientists and engineers have worked for decades to solve the organ shortage; today they are advancing along different paths to produce organs from genetically modified pigs, to “recellularize” animal organ scaffolds with human cells, and to manufacture whole-organs through 3D bioprinting.
GROWING WORLDWIDE SHORTAGE OF ORGANS
Surgery for organ transplantation is now routinely practiced in some 250 medical centers in the U.S. Thanks to improvements in immunosuppression therapies, the national one-year survival rate exceeds 90 percent for heart, kidney and liver transplants and 89 percent for lungs. In 2020, the average cost of a transplant was $442,500 for a kidney, $878,400 for a liver, and more than $1 million for a heart. Transplantation is big business, but one that is tremendously constrained by the supply of organs.
There is still a large gap between those who need and those who get transplants (Figure 1). Almost 43,000 organ transplants were performed in the U.S. in 2022, while roughly 105,000 patients were on the organ waitlist in the U.S. (Figure 2). Likely another 100,000 (or more) patients are without access to the sophisticated health care that would enable them to join the waitlist. “Those who are in the greatest need and most disadvantaged when it comes to access to adequate medical care are also those who are the least likely to get organs,” said Brendan Parent, bioethicist and Assistant Professor of Surgery at New York University (NYU). “These people might never get on the waitlist — they live in rural areas or are too sick or too poor or don’t know that a transplant is possible.” Persistent efforts to encourage organ donation and to improve the organ procurement process have, at least thus far, failed to make a dent.
Annual Number of Patients on Waiting List, Transplants Received, and Living and Deceased Donors in the United States, 1988-2018
Figure 1. The number of patients with requiring an organ transplant has grown much faster than the number of organs available. * Source: National Survey of Organ Donation Attitudes and Practices, 2019. U.S. Department of Health and Human Services Health Resources and Services Administration.
Figure 2. The organ waitlist in the U.S. has over 100,000 candidates; and likely another 100,000 with organ failure who are not on the waitlist. Despite options for dialysis and living donor kidneys, there are still nearly 90,000 candidates waiting for a kidney. * Source: “Organ donations and transplants in the U.S.,” Statista, 2022, Article number: did-26138-1, 24.
*A candidate who is waiting at more than one center, or for multiple organs, would be counted as only one candidate.
Roughly 38,000 organ transplants were performed in Europe in 2021; absent the COVID pandemic, this number would likely have been closer to 50,000 (Figure 3). Transplant activity in developing regions has been growing rapidly; between 2012 and 2021, the number of organ transplants in India grew 4.3 times. In the ten years prior to the start of the COVID pandemic, transplants in China and Brazil increased by roughly 2.6 and 1.5 times, respectively. The World Health Organization (WHO) estimates that the organ supply is less than 10 percent of the global need, underscoring the urgency for new approaches to ease the acute shortage.
Figure 3. Transplants by country (sum of kidney, heart, lung, liver, pancreas, small bowel), 2021. * Source: Global Observatory on Donation and Transplantation
Today interdisciplinary teams of researchers are leveraging advances in stem-cell technology, cloning, bio reactors, gene editing, 3D bioprinting, and material and computer science to pursue innovative ways to produce organs. “Off-the-shelf” organs may still be a decade or more away, but the potential benefits of applying these widely-used technologies to the production of organs could be great. With scale, marginal costs would likely fall rapidly, allowing for organs of high and uniform quality to be produced economically through an industrial process. What’s more, the research projects currently underway could lead to organs that are produced from compatible cells or even from a patient’s own cells, presenting little or no risk of rejection and reducing the side-effects and significant cost of immunosuppression drugs.
TISSUE-ENGINEERING
In 1993, MIT professor Dr. Robert Langer (one of the co-founders of Moderna) and Dr. Joseph Vacanti, Professor of Surgery at Harvard Medical School, decried that “the loss of an organ or tissue is one of the most frequent, devastating and costly problems in human health care.” They envisioned an approach to solve the organ shortage through “tissue engineering” — “an interdisciplinary field that applies the principles of engineering and the life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function.”
Over the past three decades, advancements in tissue engineering include the creation of skin, bone, intestine and cartilage tissues, and lab-grown bladders. Tissue-engineered skin has been grafted onto burns and wounds. In June 2022, a young woman born without a complete earlobe received a 3D printed ear comprised of her own cells. Dozens of tissue engineering research labs have sprung up in leading universities. Researchers at Wake Forest, where lab-grown pediatric bladders were first produced and transplanted into pediatric patients, claim to be pursuing regenerative therapies for 40 organs and tissues. Carnegie Mellon's Bioengineered Organs Initiative is working on advanced 3D bioprinting techniques and novel biomaterials; Johns Hopkins researchers are modeling and synthesizing blood vessel structures; Stanford scientists are exploring the potential to develop human organs with animals as hosts. While the flurry of competing institutions taking different approaches is encouraging, Dr. Langer cautions that creating complex organs for transplantation still faces significant technical hurdles.
Dr. Vacanti, now head of the Tissue Engineering and Organ Fabrication Laboratory at Massachusetts General Hospital (MGH), has worked to solve the organ shortage for three decades. He remains hopeful: “Our goal is to create a completely living replacement liver structure without artificial parts.” Dr. Vacanti’s lab is pursuing a staged approach: “We’re first building a partial liver structure, not a complete organ, so that we can validate our core ideas and technology. In order to build something big enough that’s living, it needs to have its own blood supply, and we have now built those circulations. We’ve spent the last 24 years on this; it has required an enormous amount of effort and the expertise of a huge team of computational dynamics experts.”
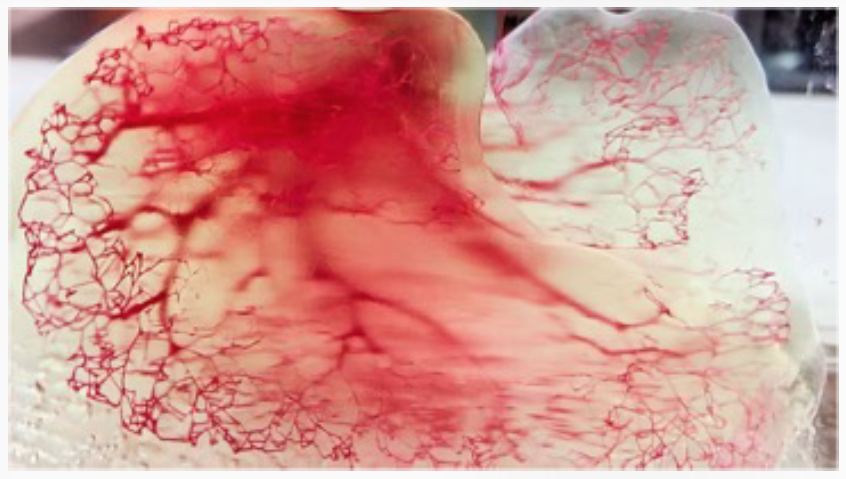
To create vascular networks for engineered tissues, Dr. Vacanti’s team has applied the mathematical concept of fractals — geometric structures whose smaller parts resemble the whole at multiple levels of scale — such as trees, ferns, snowflakes, to the human nervous and vascular systems. Blood vessels are a complex example of fractal branching where the branches get smaller and smaller down to a tiny capillary. Dr. Vacanti explained that “once you know the algorithm of a certain fractal, you can scale it infinitely; that’s what we are doing when building fractal-based vascular networks with 3D bioprinters.” (Bioprinting is a 3D printing technology used to make functional replacements for damaged or diseased tissue using “bioink,” a solution that has the properties of the target tissue.) In Dr. Vacanti’s lab, bioprinters produce liver implants that contain a vascular set of channels designed to mimic portal flow and which can be used for partial or full liver function (Figure 4).
Figure 4. 3D printed model of a tissue engineered human liver demonstrating vascular channels in red and blue, and liver and biliary tissue in green. * Source: Courtesy of JP Vacanti
The production of these liver implants involves the bioprinting of scaffolds that are loaded with the recipient animal’s own cells (eliminating the potential for rejection) and then incubated in flow conditions in culture. After a few days, the implant is surgically inserted into a pig. Dr. Vacanti said “Conceptually, it’s just like a transplant. We hook it up to the portal inflow so that we have portal blood flowing into the implant and then we connect it to the outflow of the liver to the heart.” The team’s recent tests in pigs confirm that cells in the bioprinted liver implant remained alive and that the implant performed liver-specific functions.
The next big hurdle is whether Dr. Vacanti’s lab can print organs with enough function per cubic centimeter of mass to replace a whole liver. “We know we can provide liver-specific function,” he said, “but we don’t yet know if it is of sufficient quantity. I think I see a straight shot...I’m pretty confident that we can generate an organ that has 10 times the function of what we have now, but the biggest question is what is big enough? How close can we get to the compact functionality of a human liver?”
Although hesitant to specify when his lab might produce a complete tissue-engineered liver, Dr. Vacanti sees smaller but significant wins along the way. For example, many liver transplants are the result of a sudden shock to a healthy liver, such as an overdose of Tylenol, acute hepatitis, or shellfish poisoning. In such cases, an enhanced version of the 3D bioprinted implant might help to avoid a liver transplant, saving significant risk and cost to the patient. Since the liver is a regenerative organ, the implant could be temporarily implanted or even connected outside the body to perform the liver functions while the damaged liver repairs itself.
The big win will come if bioprinting of organs becomes scalable. “Early on Bob [Langer] and I turned to synthetic scaffolding systems because we wanted a process that was manufacturable,” said Dr. Vacanti. “Our technology is a platform for building large living structures, and a 3D bioprinter could conceivably shape any organ, or extremities, or complete faces.” A platform approach, if successful, could dramatically increase the supply and reduce the marginal production cost of organs for transplant.
United Therapeutics: “Multiple Shots at Goal”
United Therapeutics (UT) develops novel pharmaceutical therapies as well as technologies to address the acute shortage of organs for transplant. Its CEO, Dr. Martine Rothblatt, previously the founder of SiriusXM radio, was motivated to find therapies for pulmonary arterial hypertension (PAH), a disease that her daughter was diagnosed with as a child. UT’s long-term goal is to develop both porcine and tissue-engineered organs, including lungs for patients with advanced PAH who are likely to require a lung transplant. Dr. Rothblatt has stated that the company’s vision “is to manufacture organs as a way of increasing the supply so that more people can get one instead of dying.” UT wants “to create an autologous organ from the patient’s own cells so that they can have an organ that they can live with for life.”
Currently focusing on lungs, hearts, and kidneys, UT is pursuing three different approaches to solving the organ shortage. “You might call it multiple shots at goal,” said Dr. Luis Alvarez, UT Vice President of Organ Manufacturing. For xenotransplantation, UT’s Revivicor subsidiary produces genetically-modified pigs to produce human-compatible organs, such as the hearts and kidneys used in recent experimental procedures. Second, is a “decell/recell”approach which involves the decellularization (removal of cells) from porcine (pig) organs, currently lungs, followed by the recellularization of the remaining scaffold with allogenic cells (meaning from the “other”) of a human whose gene type is compatible with the intended recipient patient. The optimal decell/recell process would use “autologous” cells of the patient receiving the organ. Another option is refurbishing cadaver lungs that would have otherwise been rejected for transplant, a process known as ex-vivo lung perfusion (EVLP). Finally, organ manufacturing, the creation of whole organs through the use of new biomaterials, cell technologies, and bioprinting, might realize the goal of off-the-shelf organs. Each approach has unique advantages and risks with respect to the feasibility of production and rejection by the immune system; if successful and eventually approved for clinical use, they could the ease the organ shortage and potentially improve patient outcomes by allowing more control over the timing of a transplant.
Xenotransplantation: Genetically-Modified Pigs as Organ Donors
Like Drs. Langer and Vacanti, Dr. David Ayares, President and chief scientific officer of Revivicor, has been pursuing a solution to the organ shortage for several decades. In March 2000 while working for PPL Therapeutics (the company that cloned Dolly the Sheep), Dr. Ayares led the project that produced the first cloned piglets. As he explained at the time, “The birth of these piglets is a very significant accomplishment…it has the potential to essentially revolutionize the transplant field.” Back then, they were hopeful clinical testing in humans might be just four years away.
Pigs are attractive as a source of organs because they share anatomical and physiological traits with humans and their organs are of an appropriate size. However, to avoid rejection of pig organs in humans, pigs must be genetically modified. The chief culprit is the “alpha-gal” — galactose alpha 1-3 galactose, a sugar molecule in pigs that is immediately recognized as foreign and attacked by the human immune system. By 2003, Dr. Ayares’ team had combined cloning with the “knockout” of the alpha-gal gene to create “GalSafe” pigs. During tests in baboon and rhesus models, the Revivicor team identified other antigens that could cause human rejection. Eventually, a total of 10 genetic modifications were performed: three knockouts to prevent rejection (the disabled alpha-gal and two others) and an additional knockout to prevent the organ from growing too large for a human recipient. Six “knock-ins” (additions) of human genes further reduce the likelihood of rejection by preventing abnormal blood clotting, inflammation and attack by antibodies.
In January 2022, Revivicor provided the heart of one of its genetically-modified pigs for xenotransplantation into David Bennett, a 57-year-old patient at the University of Maryland at Baltimore (UMB) suffering from end-stage cardiac failure. Since Mr. Bennett was not eligible for a transplant of a human cadaver heart, the FDA granted the UMB transplant team, emergency approval for the procedure, the first of its kind (Figure 5). Mr. Bennett died two months later; the cause of his death is not certain though doctors have cited a number of possible factors. The pig heart retained fluid, increased in size and stopped functioning; the immunosuppression regimen was interrupted, possibly causing rejection. Cell-free DNA for porcine cytomegalovirus (PCMV) was detected, but it did not infect Mr. Bennett, and it is not known if it contributed to the failure of the heart. Dr. Richard Pierson, a cardiac surgeon at MGH and scientific director of the MGH Center for Transplantation Sciences, is hopeful that managing PCMV in organ recipients is a solvable problem and that xenotransplants could help solve the organ shortage. Dr. Pierson noted that “given how sick Mr. Bennett was before the transplant, that he lived two months after the transplant is revolutionary and remarkable.”
Figure 5. “University of Maryland School of Medicine Faculty Scientists and Clinicians Perform Historic First Successful Transplant of Porcine Heart into Adult Human with End-Stage Heart Disease.” For a fascinating look into the operating room, watch here. * Source: University of Maryland
In further research during the summer of 2022, surgeons at NYU transplanted pig hearts supplied by Revivicor into two brain-dead patients maintained on ventilators. The three-day studies included more rigorous protocols for the assessment of pig viruses as well as data collection on the performance of the transplanted hearts. Revivicor envisions that its modified pigs can be also be a source of kidneys; in late 2021, NYU surgeons performed two xenotransplants of Revivicor GalSafe pig kidneys into deceased humans maintained on a ventilator. NYU reported that in both operations, the kidney functioned properly for 54 hours without exhibiting rejection.
While there is no concrete timetable, these recent xenotransplant studies anticipate human clinical trials on a larger number of human patients. In June of 2022, an FDA official indicated that “Advances in understanding xenotransplant rejection and technologies enabling the genetic modification of pigs for xenotransplantation have moved the field closer towards initiating clinical trials.” At the same time, the FDA indicated that “many questions remain regarding the transmission of infectious disease, the effects of the genetic alterations…on the pigs, and the use of immunosuppression in xenotransplant patients.”
Revivicor’s parent company, UT, is constructing a “Designated Pathogen-Free” (DPF) facility in Virginia to produce more of its genetically modified pigs for eventual clinical trials. Before receiving approval for phased human clinical trials, Revivicor and medical researchers will progress along the FDA regulatory path — collecting extensive data, demonstrating consistent survival in non-human primate studies, and performing further experimental procedures. Dr. Ayares noted that this will require a significant amount of funding, with each clinical trial costing as much as $1.2 to $1.5 billion. During this period, other issues must be resolved, such as how many genetic modifications are really necessary for each type of organ and whether the genetically modified pigs can be produced at scale. If the pre-clinical trial work goes smoothly, clinical trials might commence in 2-3 years around the time the new DPF facility is completed.
“Decell/Recell” of Porcine Organs: A Moonshot?
Decell/recell involves taking an animal organ, such as a pig’s lung, and removing (decellularizing) the pig cells and then replacing them (recelluarizing) with human cells. UT has developed an automated approach in which pig lungs are placed in a controlled bath with specially-formulated solutions and, over several dozen hours, the pig matter — cells and biomass — is removed from the lung leaving only the collagen scaffold. The company is using these scaffolds to test and refine the recellularization process in the hope that they might become a supply of ULobe™ transplantable lungs (Figure 6).
Figure 6. United Therapeutics’ ULobe™ (pre-clinical) is a pig lung scaffold decelluarized and repopulated with human allogeneic cells. * Source: United Therapeutics
While removing all of the pig matter may circumvent inflammatory rejection, there are difficult challenges in producing lungs with the decell/recell process. Since the most important function of the lung is gas exchange, it is critical for the added cells to seal any gaps or holes that may arise during the decell process. Dr. Pierson from MGH explained: “Unlike livers or kidneys, or even hearts, the lung has very little tolerance for error. If just one percent of the lung blood vessel surface area is leaking, 100 percent of the lung fills up with blood and it cannot function.”
An alternative approach is the creation of a hybrid decell/recell organ. Rather than trying to remove all the pig cells from the pig lung, just the pig’s endothelial cells (cells that form the lining of blood vessels) could be removed. Human endothelial cells would then be added to this scaffold. “As long as the cells lining the blood vessels are human, and the pig has been genetically modified to prevent an immune response from a human, it may not matter that the other lung cells are from a pig,” explained Dr. Pierson.
Ex-Vivo Lung Perfusion—a Stopgap Solution?
Many lungs from cadaver donors are discarded because they are not of sufficient quality to be transplanted. UT has established a Centralized Lung Evaluation System (CLES), a platform and service currently under clinical trial, to examine discarded lungs and then refurbish those which can be made suitable for transplant. Performed in specialized facilities in Jacksonville, F.L. and Silver Spring, M.D., ex-vivo lung perfusion (EVLP) is a process in which a cadaver lung is a “patient” and examined, perfused and treated with a solution that delivers nutrients, proteins and oxygen (Figure 7). Refurbished lungs with proper function are then sent to participating transplant centers. As of October 2022, UT states that more than 250 EVLP lungs have been successfully transplanted to patients with end-stage lung disease.
Figure 7. Cadaver lungs undergoing ex-vivo lung perfusion (EVLP) in UT’s Centralized Lung Evaluation System, currently in clinical trials. To see the EVLP process, watch here. * Source: United Therapeutics
The Future: Organ Manufacturing through 3D Bioprinting?
While significant technical hurdles remain, the biomanufacturing of whole organs might offer the most promising long-term solution to the organ shortage. Dr. Luis Alvarez, UT Vice President of Organ Manufacturing, claimed that “one major benefit of our approach is we’re not dependent on an animal source for the scaffold.” Dr. Alvarez explained that the scaffold material must be compatible with the human body, very favorable for cellularization, and printable at very high resolution. “Our scaffold material is one of the most closely guarded aspects of the scaffold,” he said, “it is thecompetitive factor.”
Dr. Alvarez described the organ production process under development at UT: first, a scaffold to hold the cells is bioprinted (Figure 8); some currently take days to print, some only a couple of hours. “We’re working to reduce the print time; within a short time, we'll likely know how long it will take to print a clinical quality lung.” UT is collaborating with 3D Systems to design and manufacture “the most capable and the highest-resolution 3D bioprinters anywhere,” said Dr. Alvarez. The challenge is to achieve very high precision, much like that required in the semiconductor industry to produce computer chips. Borrowing certain manufacturing techniques from the semiconductor industry may allow companies like UT to enjoy a “tailwind” in bioprinting cost and scalability, thus making organ production more cost effective over time.
Figure 8. United Therapeutics’ ULung™ (pre-clinical) is a personalized lung composed of a 3D printed lung scaffold that is populated with either allogenic cells (from a third party) or with autologous cells (from the patient). * Source: United Therapeutics
Populating the bioprinted scaffolds with human cells is more complicated. Deriving induced pluripotent stem cells (iPSCs) from a patient’s own cells might require a couple of weeks. Differentiating those iPSCs into specific cell types could add another month. Dr. Alvarez said differentiating iPSCs into vascular cells is well established, and UT is working on the process to differentiate airway cells for lungs.
“One approach is to start the cell work along with the scaffold printing and have the two converge at the first moment possible,” explained Dr. Alvarez. “We can pre-print and keep an inventory of the scaffolds; they are shelf-stable, so we might print various sizes and store them until needed.” He believes that the bioprinting will not be the bottleneck and that the cell generation is solvable. “To date, scientists have managed to figure out how to make many, many cells in the body from iPSCs, and so I think of it as more of an engineering problem than a science problem.”
Each organ has its own engineering challenges. “The lung is a large organ with a lot of fine features,” Dr. Alvarez. “In some ways, it's probably the most challenging organ. The kidney is much smaller, so you can print multiple kidneys in the same footprint of a single lung. But a kidney also has some pretty fine features. A big challenge with livers is getting the liver cells that are inserted in a manufactured scaffold to ‘feel at home’ in their new environment and perform their function.”
In June 2022, Dr. Martine Rothblatt and Chuck Hull, co-founder of 3D Systems, showed the first 3D printed lung scaffold, claiming that it is “world’s most complex 3D-printed object,” consisting of 44 trillion voxels (think 3D pixels) that form “4,000 kilometers of pulmonary capillaries and 200 million alveoli.” 3D Systems is also developing 3D printed livers and kidneys (Figure 9).
Figure 9. Demonstration of 3D printed lung at the CNN LifeItself conference on June 6, 2022. * Source: United Therapeutics
In the future, Dr. Rothblatt envisions “a giga-factory for organs” in a clean-room environment with highly-automated printers making scaffolds that move along each step of the assembly process, adding cells, maturing the organs, and placing them in a perfusion cart for delivery — ultimately resulting in an unlimited supply of compatible organs. Dr. Alvarez commented “Although we could use allogenic cells, we're aiming for the autologous approach using cells from the patient. Then we would have truly-matched organs and an unlimited supply of them. That’s the ultimate solution to the organ shortage.” Dr. Vacanti shares this view: “If you can give patients an organ with their own cells, not only have you solved the organ shortage problem, you’d also solve the immune problem.”
CONCLUSION
The current transplant era was enabled by the introduction of immunosuppression drugs, notably the approval of cyclosporine in 1983, that allowed patients to live with a transplanted organ, though often with serious side effects. This gave hope for many but was accompanied with the anxiety of waiting for a suitable organ and the risk of tissue rejection. Since then, improvements in surgical techniques and antirejection drugs have allowed more than a million patients in the U.S. to receive transplanted organs. The need for organs far outpaces the supply and the gap continues to widen. The production of suitable animal donor organs along with the advances in tissue engineering and 3D bioprinting may unlock new supplies of organs that are highly compatible with the patient. If realized, these advances hold promise for the disparate patients suffering organ failure to live fuller and longer lives.
It is not clear whether pig organs, decell/recell organs, biomanufactured organs, or some hybrid approach will be the long-term winner. “After living with the organ shortage for so long, I’d support any solution that works,” said Dr. Vacanti. Assuming that the risks surrounding the transmission of pig viruses can be contained, xenotransplantation for hearts and kidneys seems the closest solution. “If I were to guess the evolution of this,” said Dr. Esquivel, “I think xenotransplant will come first, then animal scaffolds, followed by bioengineered scaffolds. We might transplant kidneys from pigs within five years.”
“I’m really bad at making predictions,” said Dr. Langer. “Handling many different cell types, getting the correct scaffold structure, having enough cells, handling rejection; these are all very complex issues. Obviously, it depends on science and regulatory issues, but then you never know…suddenly something happens that changes everything. People said messenger RNA might take a long time, but then COVID came along and it’s widely used within a year.” Dr. Rothblatt,however, is bullish: “Before the end of this decade we will develop an unlimited supply of organs…that is the purpose of my life in the 2020’s.”
One organ donor can save 8 lives. You can register to be an organ donor here.
About the Author:
Mary Sauer is a Senior Fellow in the Harvard Advanced Leadership Initiative and has extensive entrepreneurial, business scaling and seed stage investing experience. Mary is currently co-founder of a seed stage financing firm and was previously co-founder of a publicly traded software company. She currently serves as Advisor to the Harvard Future of Work Project and on the Dean’s Cabinet of the Harvard School of Engineering and Applied Sciences, the Stanford MLA Advisory Council and the Friends of Florence Advisory Circle.
***********
Acknowledgements:
A special thanks to those I interviewed for this article — Luis Alvarez of United Therapeutics, David Ayares of Revivicor, Alex Chan of Stanford, Glenn Cohen of Harvard Law School, Carlos Esquivel of Stanford, Robert Langer of MIT, James Lytle of the Harvard Petrie-Flom Center, Brendan Parent of NYU, and Joseph Vacanti of Harvard Medical School.